Commitment to Education Award

Keller Technology Corporation was selected to receive a Niagara Frontier Industry Education Council 2015 Commitment to Education Award. The Commitment to Education Award honors industry leaders who support the mission of the NFIEC and have partnered with schools to foster student success in the workplace. The award was based on a nomination received from the […]
KUKA Robotics Official System Partner

Keller Technology named a KUKA Robotics Offical System Partner. KUKA’s line of industrial and collaborative robots complement Keller Technology’s expertise in the semiconductor, life sciences, and OEM equipment industries. Keller Technology is working with KUKA to bring maximum value to customers seeking equipment solutions in those industries. Keller Technology Corporation is pleased to announce that […]
Why Protecting Intellectual Property Is Serious Business

Choose a contract manufacturing partner that will protect your intellectual property. A contract manufacturer (CM) should focus on IT security to project your IP from breaches. The contract manufacturer you select should have procedures in place to restrict entry to its facility. We’ve all heard the horror stories of a company suddenly competing against what […]
5 Things to Consider When Outsourcing the Manufacture of Medical Equipment Assemblies
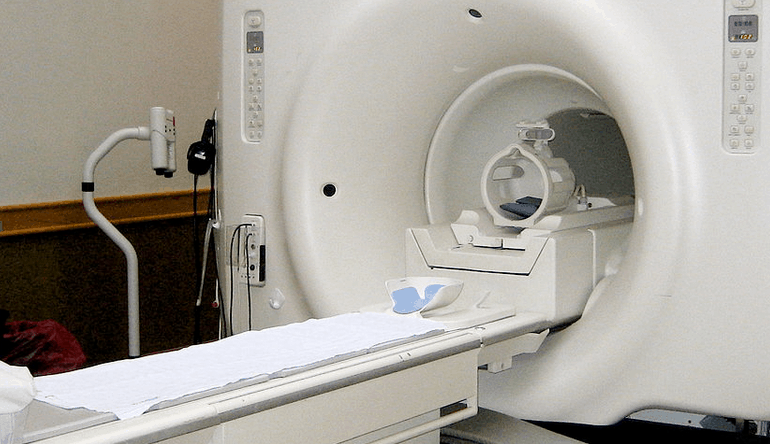
Medical device manufacturers can benefit from outsourcing high level assemblies. Contract manufacturers (CMs) should hold ISO 9001 and ISO 13485 registrations. The chosen CM should have a well-developed supply chain. For some producers in the medical industry, particularly those manufacturers of large, capital medical equipment, it may be impractical or impossible to outsource the entire […]
Does Your Contract Manufacturer Need to Be ISO 13485 Registered?

ISO 13485 registration is a necessity for medical device manufacturers. Avoid potential liability issues by partnering with a 13485-registered CM. Consider ISO 9001 registration for the manufacture of non-medical devices. There seems to be an emerging debate on the necessity of selecting an ISO 13485-registered company to handle contract manufacturing needs. Let’s begin with the […]
Three Points to Consider When Selecting a Contract Manufacturer for Medical Devices

An ISO 13485 registered contract manufacturer (CM) must regularly demonstrate existing infrastructure to encompass the traceability, risk assessment, and QMS required to support their customer’s regulatory compliance. A CM should have business systems and procedures in place for achieving and maintaining aggressive schedules for the manufacture of your particular medical device. A CM must have […]
