Build-to-Print Solutions for Medical Equipment and Medical Device Manufacturing Systems
Keller Technology Corporation offers specialized experience in building medical device manufacturing systems and complex equipment for a variety of applications. Our build-to-print services offer custom solutions for your most challenging applications.
- ISO-certified facilities including dedicated controlled environment (clean room) areas
- Strict quality management systems assure regulatory compliance
- Experienced with high-complexity equipment,robotics, and automation integration
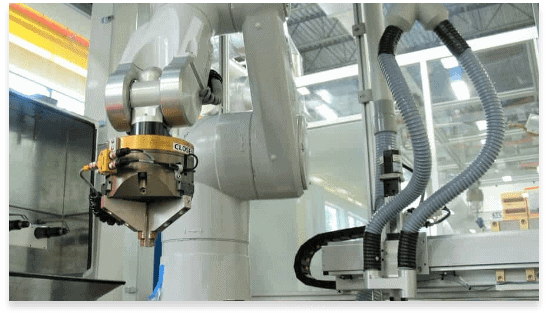
KTC’s specialized capabilities and superior support ensure your project’s success
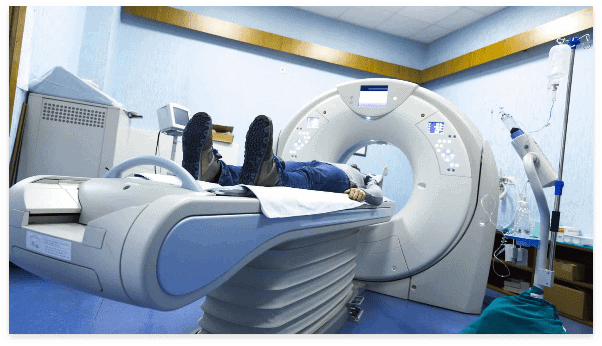
Medical equipment and medical device manufacturing systems requires technical expertise and consistent quality. KTC offers expert manufacturing of cGMP-certified custom equipment for health care OEMs, pharmaceutical/biopharmaceutical systems and subsystems, and medical device systems.
KTC’s production experience includes high-level, complex technologies such as beam delivery systems, accelerator and magnetic-based imaging equipment, proton therapy, clean-room assemblies, robotics systems, and hard automation.
Certified Compliance
To help you meet your compliance requirements, our Buffalo, NY, production facility is IS0-9001 certified and our Charlotte, NC, facility is both ISO-9001 and ISO-13485 certified. Additionally, we offer clean-room capabilities and GAMP validation.
Buffalo, NY

Charlotte, NC

Work with Keller
KTC offers advanced solutions to your most demanding medical equipment challenges.
Build-to-print frequently asked questions (FAQs)
Every build-to-print medical device systems project begins with a thorough review of all documentation. We often find potential issues, such as missing information or obsolete commercial items, which we can remedy ahead of project commencement.
KTC can take your prototype (or proof-of-principle) and create a design for manufacturing, including a complete documentation package.

